Vaccine adjuvant technology
More than 40 years’ experience with vaccination
The world population is expected to reach 9.8 billion people in 2050. The fight against infectious diseases, both in humans and animals, is one of the major challenges of global health. Some animal diseases can have a strong impact, on the animals themselves, particularly on farms, by threatening our food supply, and also by transmission to humans. To date, vaccination is one of the most effective ways of dealing with infectious diseases.
The role of vaccine adjuvants in the immune response
Vaccine adjuvants (from the Latin adjuvare meaning “to help”) are substances that increase the intensity of the immune response after co-administration with an antigen. They are useful for enabling vaccines to induce strong and persistent immune responses, while reducing the vaccine dose and number of injections, and increasing the vaccine’s stability. Adjuvants for vaccines are used in the formulation of human and veterinary vaccines.
Categories of vaccine adjuvants
In human vaccination, only few adjuvant technologies are used in the formulation of vaccines on the market. Aluminum salts have been used for over a century. New technologies have been developed in recent decades, such as liposomes, purified Quillaja saponaria saponin, and squalene-type emulsions in water.
Veterinary vaccination uses a greater variety of adjuvant technologies, in particular aluminum salts, oil-in-water, water-in-oil or multiple emulsions, polymer dispersions, saponins, as well as other naturally occurring immunostimulants.
Vaccine adjuvants can have two mechanisms of action:
- As vectors which, due to their physicochemical properties, simulate the presence of an infectious agent. These effectively present the antigen to cells of the immune system in order to facilitate its rapid absorption and improve the immune response. This is especially so with aluminum salts and emulsions.
- As immunostimulants that specifically interact with receptors located on immune cells. This is the mechanism of action in Toll-Like Receptor (TLR) ligands, for example.
Several adjuvant technologies combine these two mechanisms to potentiate their effectiveness. It is also possible to combine two different types of adjuvants within the same vaccine.
Select a vaccine adjuvant
The use of an immunity adjuvant is considered from the outset of a vaccine development project, whether human or veterinary. In developing an inactivated or subunit vaccine, it is necessary to add an adjuvant to guarantee the effectiveness of the vaccine and the duration of the immunity conferred. For the development of a live attenuated or vectorized vaccine, the addition of an adjuvant is optional, but may reduce the antigenic load per vaccine dose.
An adjuvant is expected to be:
- Effective and capable of inducing – in combination with the selected antigen – a strong immune response to protect the host from an infectious disease,
- Well-tolerated so as not to cause a local or systemic reaction,
- Compatible with the antigen in the formulation to allow the formulation of a stable and reproducible vaccine,
- Easy to use, both for formulation with the antigen and for injection,
- Reproducible in order to guarantee the same performance independently of the batch, and produced according to the quality benchmark required for the desired application.
There is no universal adjuvant that is suitable for all pathologies. The selection of an adjuvant for a vaccine project needs to integrate many criteria, in particular the type of vaccine (prophylactic or therapeutic), the mode of administration, the antigen technology, the immune orientation of the response sought for the pathology target, the duration of immunity sought, and, for animal health, the target animal species.
Seppic develops and offers various vaccine adjuvant technologies dedicated to human and animal health, in particular the oily and polymeric adjuvants of the MontanideTM range. Seppic has also developed the required expertise to select the most suitable adjuvant for each vaccine development project.
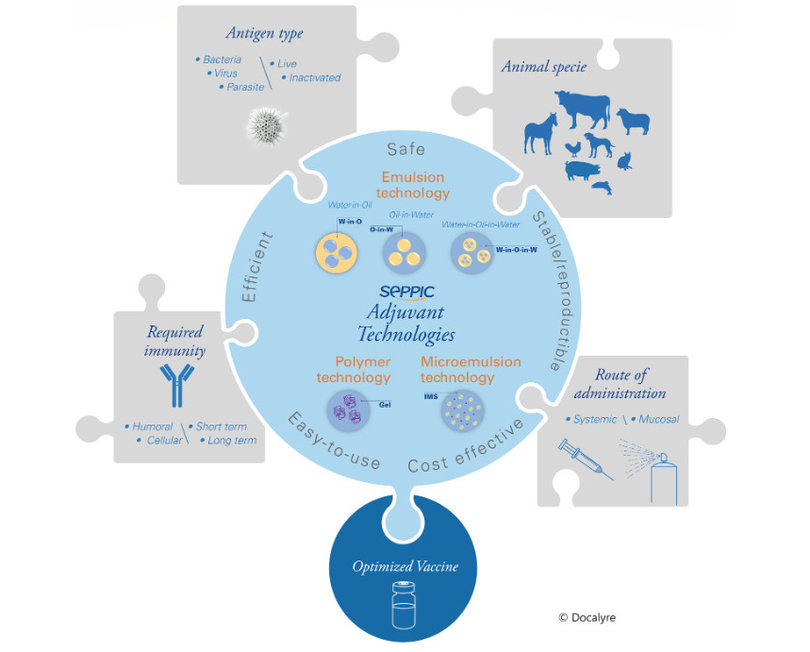
Emulsion adjuvant technology
Oily adjuvants allow the formulation of vaccine emulsions in the presence of an aqueous phase containing the antigen. These emulsions can be of different types: oil in water, water in oil, or multiple water in oil in water. These different types have different safety and efficacy profiles and are adapted, in animal health, to different animal species and different types of antigens.
Seppic develops and markets ready-to-use oily vaccine adjuvants for human and animal vaccination to formulate emulsion-type vaccines. These adjuvants consist of an oily phase and surfactants, selected and precisely characterized to guarantee the stability of the adjuvants, as well as the stability, efficacy and safety of the vaccines formulated with these adjuvants.
The fatty phase can consist of an oil from a mineral, vegetable or synthetic origin, or of a combination of these different oils. The surfactant is synthesized and selected for each adjuvant by optimizing its hydrophilic-lipophilic balance, depending on the type of emulsion sought and the fatty phase selected. To make this selection, we rely on our expertise in chemistry and analysis of surfactants, on a long experience in formulation and galenic characterization of vaccine emulsions, and on the methods of characterizing the post-vaccination immune response developed in our immunology laboratory.
To obtain a vaccine emulsion, these adjuvants are then emulsified in the presence of an aqueous phase containing the antigen according to a dedicated manufacturing process that Seppic is developing for each of its adjuvants. This emulsification process is transposed from the laboratory scale to the pilot and industrial scale, which allows us to support the industrial transposition of our customers.
Our adjuvants are developed and then tested on a pilot scale in our Innovation laboratories in Castres, before being transposed for industrial scale in our production workshops dedicated to the manufacture of adjuvants. We manufacture human and veterinary vaccine adjuvants at our Castres site (France) and, for animal health, at our Qing Pu site (China).







